Difference between revisions of "Lead acid battery"
| Line 12: | Line 12: | ||
This page will focus on flooded lead acid (FLA) and valve regulated lead acid (VRLA) deep-cyle batteries. | This page will focus on flooded lead acid (FLA) and valve regulated lead acid (VRLA) deep-cyle batteries. | ||
| − | |||
| − | |||
| − | + | ==Flooded lead acid (FLA)== | |
The original lead acid battery design. They are the simplest, most durable and cheapest of the lead acid batteries. They are slightly more durable as they tend to be more forgiving of deep discharges than maintenance-free VRLA batteries. | The original lead acid battery design. They are the simplest, most durable and cheapest of the lead acid batteries. They are slightly more durable as they tend to be more forgiving of deep discharges than maintenance-free VRLA batteries. | ||
| − | Characteristics: | + | ====Characteristics:==== |
*They require monthly maintenance as the batteries will lose water as they charge and discharge. Durable, long cycle life and cheap only if properly maintained. If maintenance is not performed regularly, the batteries will rapidly fail and replacement will cost significantly if maintenance-free batteries had been used initially. | *They require monthly maintenance as the batteries will lose water as they charge and discharge. Durable, long cycle life and cheap only if properly maintained. If maintenance is not performed regularly, the batteries will rapidly fail and replacement will cost significantly if maintenance-free batteries had been used initially. | ||
*Release significant amounts of hydrogen gas. | *Release significant amounts of hydrogen gas. | ||
| Line 24: | Line 22: | ||
*Can undergo an [[Charge controller#Equalization charge|equalization charge]] which can help to prolong their [[Energy storage#Cycle life|cycle life]]. | *Can undergo an [[Charge controller#Equalization charge|equalization charge]] which can help to prolong their [[Energy storage#Cycle life|cycle life]]. | ||
| − | Considerations for use: | + | ====Considerations for use:==== |
*End users that are capable of maintaining a battery (with proper training and protective equipment) or in a location where the system can be serviced by technicians. | *End users that are capable of maintaining a battery (with proper training and protective equipment) or in a location where the system can be serviced by technicians. | ||
*Users must have reliable access to distilled water was any other form of water has impurities and will damage the battery. | *Users must have reliable access to distilled water was any other form of water has impurities and will damage the battery. | ||
| Line 31: | Line 29: | ||
*Low budget and high energy needs. | *Low budget and high energy needs. | ||
| − | + | ==Valve-regulated lead acid (VRLA)== | |
In the 1970's maintenance-free lead acid batteries began to enter the market. These batteries are designed to address several of the primary concerns that arise with FLA batteries. They are less durable, have a shorter cycle life and cost significantly more than flooded lead acid batteries. They are less foriving of deep discharges. There are two primary sub-categories of VRLA batteries: AGM and Gel. | In the 1970's maintenance-free lead acid batteries began to enter the market. These batteries are designed to address several of the primary concerns that arise with FLA batteries. They are less durable, have a shorter cycle life and cost significantly more than flooded lead acid batteries. They are less foriving of deep discharges. There are two primary sub-categories of VRLA batteries: AGM and Gel. | ||
| − | Characteristics: | + | ====Characteristics:==== |
*They do not require maintenance. | *They do not require maintenance. | ||
*They are sealed (although not completely) so there is little to no offgassing. | *They are sealed (although not completely) so there is little to no offgassing. | ||
*They do not have a liquid electrolyte solution inside and are additionally sealed to prevent leaks, therefore they do not have to be positioned upright at all times. | *They do not have a liquid electrolyte solution inside and are additionally sealed to prevent leaks, therefore they do not have to be positioned upright at all times. | ||
| − | Considerations for use: | + | ====Considerations for use:==== |
*End users that may not perform maintenance. | *End users that may not perform maintenance. | ||
*Locations in which there is not a seperate space that can be dedicated to energy storage. | *Locations in which there is not a seperate space that can be dedicated to energy storage. | ||
*Require a higher budget. | *Require a higher budget. | ||
| − | + | ===Absorption glass mat (AGM)=== | |
A VRLA battery in which the electrolyte solution is contained within mats of fine glass fibers. These batteries cost on average 1.5-2 times as much as FLA and have a shorter cycle life when compared to a properly maintained FLA battery. | A VRLA battery in which the electrolyte solution is contained within mats of fine glass fibers. These batteries cost on average 1.5-2 times as much as FLA and have a shorter cycle life when compared to a properly maintained FLA battery. | ||
| − | Specific considerations for use: | + | ====Specific considerations for use:==== |
*Perform better than FLA and gel batteries in cold environments. FLA batteries can freeze and be damaged during charging/discharging. | *Perform better than FLA and gel batteries in cold environments. FLA batteries can freeze and be damaged during charging/discharging. | ||
| Line 53: | Line 51: | ||
A VRLA battery in which the electrolite solution is made into a gel paste. Gel batteries are the most costly lead acid option. They prefer slow charging and discharging, which is not ideal for renewable energy systems. | A VRLA battery in which the electrolite solution is made into a gel paste. Gel batteries are the most costly lead acid option. They prefer slow charging and discharging, which is not ideal for renewable energy systems. | ||
| − | Specific considerations for use: | + | ====Specific considerations for use:==== |
*Perform better than AGM and FLA batteries in hot environments. | *Perform better than AGM and FLA batteries in hot environments. | ||
Revision as of 06:11, 2 October 2020
Lead acid batteries rely on reversible chemical reactions between lead and acid to provide energy when it is needed and to store enegy when it is being produced. They have been around for over 150 years and have proven their durability, low-cost, recyclability, and performance under variable conditions to the point that nearly every automobile on the planet relies on a lead acid battery to start and run. Lead acid batteries have been the preferred form of energy storage for off-grid PV systems since they first began being built for the same reasons, thus most off-grid PV components are built for use with lead acid batteries in 12V, 24V or 48V configurations. Lead acid batteries have the added advantage of coming in a variety of voltages (2V, 6V, 12V) and amp-hour ratings (5Ah to 5000+Ah). But due to their high density of lead, this batteries are extremely heavy. A 12V, 225Ah flooded lead acid battery weighs around 60kg, which is approaching the upper-limit of what is easily movable without equipment.
Just like how a Solar PV module is comprised of various different PV cells connected together in series that each produce a certain voltage, lead acid batteries are composed of series connected cells with each one producing around being having a nominal voltage of roughly 2V. This means that a 12V battery will be comprised of 6 cells.
There are many different types of lead acid batteries, but they can initially be divided into two categories: starter batteries and deep-cyle batteries.
- Starter batteries are used in cars and are intended to provide large amounts of power for short periods of time with a shallow depth of discharge. They work well for this purpose, but they are unable to continuously supply power beyond a shallow depth of discharge without severely shortening their cycle life. These batteries will fail prematurely in a PV system and are not worth investing in.
- Deep-cycle batteries have a more robust design that enables them to continuously supply high amounts of power to a deeper depth of discharge. These batteries are heavier and cost more than starter batteries, but are the appropriate battery for use with a PV system.
This page will focus on flooded lead acid (FLA) and valve regulated lead acid (VRLA) deep-cyle batteries.
Contents
Flooded lead acid (FLA)
The original lead acid battery design. They are the simplest, most durable and cheapest of the lead acid batteries. They are slightly more durable as they tend to be more forgiving of deep discharges than maintenance-free VRLA batteries.
Characteristics:
- They require monthly maintenance as the batteries will lose water as they charge and discharge. Durable, long cycle life and cheap only if properly maintained. If maintenance is not performed regularly, the batteries will rapidly fail and replacement will cost significantly if maintenance-free batteries had been used initially.
- Release significant amounts of hydrogen gas.
- Have a liquid electrolyte solution inside that requires that they remain upright.
- Can undergo an equalization charge which can help to prolong their cycle life.
Considerations for use:
- End users that are capable of maintaining a battery (with proper training and protective equipment) or in a location where the system can be serviced by technicians.
- Users must have reliable access to distilled water was any other form of water has impurities and will damage the battery.
- Require a secure space with proper ventilation due to the combined hazard of the batteries spilling acid and their tendency to realease significant amounts of hydrogen gas.
- May not be the best battery type with either extreme high or low temperatures.
- Low budget and high energy needs.
Valve-regulated lead acid (VRLA)
In the 1970's maintenance-free lead acid batteries began to enter the market. These batteries are designed to address several of the primary concerns that arise with FLA batteries. They are less durable, have a shorter cycle life and cost significantly more than flooded lead acid batteries. They are less foriving of deep discharges. There are two primary sub-categories of VRLA batteries: AGM and Gel.
Characteristics:
- They do not require maintenance.
- They are sealed (although not completely) so there is little to no offgassing.
- They do not have a liquid electrolyte solution inside and are additionally sealed to prevent leaks, therefore they do not have to be positioned upright at all times.
Considerations for use:
- End users that may not perform maintenance.
- Locations in which there is not a seperate space that can be dedicated to energy storage.
- Require a higher budget.
Absorption glass mat (AGM)
A VRLA battery in which the electrolyte solution is contained within mats of fine glass fibers. These batteries cost on average 1.5-2 times as much as FLA and have a shorter cycle life when compared to a properly maintained FLA battery.
Specific considerations for use:
- Perform better than FLA and gel batteries in cold environments. FLA batteries can freeze and be damaged during charging/discharging.
Gel
A VRLA battery in which the electrolite solution is made into a gel paste. Gel batteries are the most costly lead acid option. They prefer slow charging and discharging, which is not ideal for renewable energy systems.
Specific considerations for use:
- Perform better than AGM and FLA batteries in hot environments.
Storage capacity
The storage capacity of a lead acid battery is measured in Amp-hours. The amount of this energy that is actually usable energy upon:
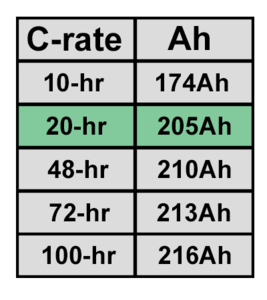
- The rate at which energy is withdrawn. If a battery lead acid battery is discharged rapidly, the amount of usable energy decreases. Conversely, if discharged slowly, the usable energy increases. This is measured in in terms of C-rate. A C-rate of 1 means that the entire capacity of the battery is discharged in 1 hour. A C-rate of 20 or C/20 means that the entire capacity of the battery is discharged over the course of 20 hours. Lead acid batteries are typically rated by their C/20 rate.
- The chosen depth of discharge limit for a battery. The cyle life of a battery depends greatly upon how deeply it is discharged and how frequently. The numbers of cycles vary depending upon the type of battery as well.
- The temperature of the battery. The temperature of the battery will affect the usable energy of a battery. Hotter tempatures increase the usable capacity of a battery, but greatly shorten the cycle life of the battery. For lead acid batteries it is estimated that for every 10°C increase in average temperature above 25°C shortens the batteries life by half. This means that operating a lead acid battery for one month at 35°C is equivalent in terms of battery life to operating the battery for two months at 25°C[1].
C-rate charge for a Trojan 12V 205Ah AGM battery[1]
Temperature
Recyclability
Lead acid batteries contain lead and acid, both of which are hazardous materials that must be disposed of properly[5]. Lead acid batteries are often pointed to as a success story for recycling as the majority of lead is used for batteries and an estimated 95-96% is ultimately recycled[6]. This largely has to do with a well-developed market, supply chain and abundant processing facilities for lead acid batteries as lead is a valuable material and is readily recyclable. This knowledge has spread and has resulted in batteries being returned for cash in even the most remote places.
Notes
- ↑ 1.0 1.1 Specifications sheet for Trojan 12V 205Ah AGM battery https://www.trojanbattery.com/pdf/SAGM_12_205_AGM_DS.pdf
- ↑ 2.0 2.1 Specifications sheet for Trojan FLA batteries https://www.trojanbattery.com/pdf/Signature_Trojan_ProductLineSheet.pdf
- ↑ 3.0 3.1 Specifications sheet for Trojan AGM batteries https://www.trojanbattery.com/pdf/AGM_Trojan_ProductLineSheet.pdf
- ↑ 4.0 4.1 Specifications sheet for Trojan Gel batteries https://www.trojanbattery.com/pdf/GEL_Trojan_ProductLineSheet.pdf
- ↑ GIZ report on End-of-Life Management of Batteries in the Off-Grid Solar Sector https://www.giz.de/de/downloads/giz2018-en-waste-solar-guide.pdf
- ↑ United Nations Enviromental Program report on recycling metals https://wedocs.unep.org/bitstream/handle/20.500.11822/8702/Recycling_Metals.pdf?sequence=1&isAllowed=y